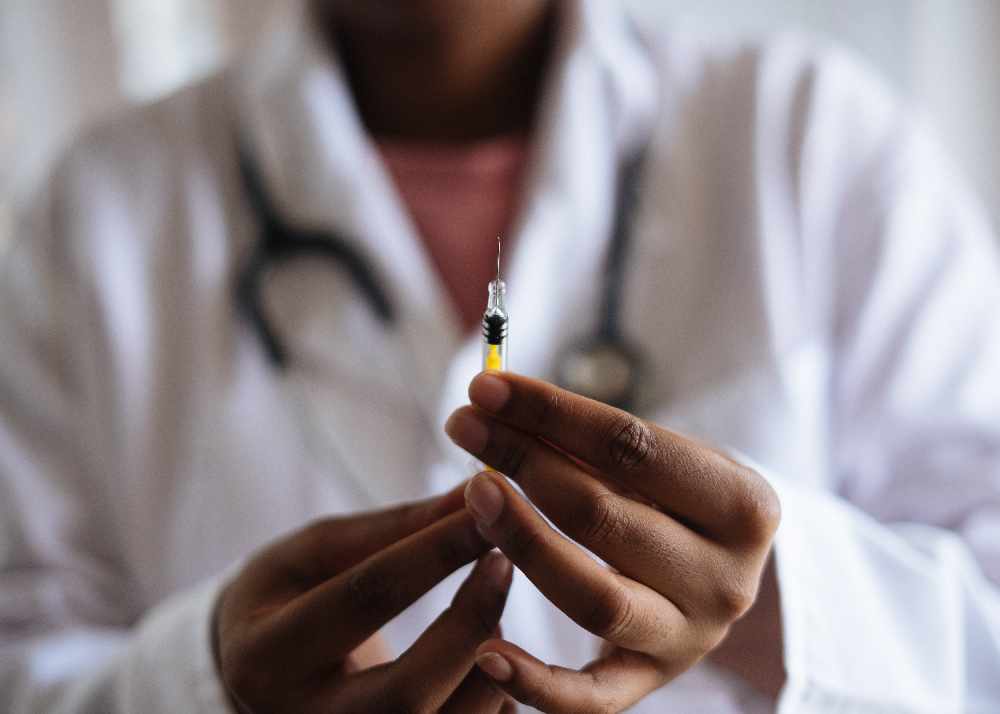
Moderna says it's going to seek authorization from health regulators to administer its COVID-19 vaccine to kids under the age of 6.
NORTH AMERICA/EUROPE - Moderna would like to receive approval to administer its COVID-19 vaccine to kids under the age of six.
It says the vaccine works for babies, toddlers and pre-schoolers and it's going to seek authorization from health regulators in North America and Europe. If it's approved, young children could receive the vaccine this summer.
Health Canada just recently approved the two-dose vaccine for the six to 11 age group. Moderna had sent in that request back in November.
The National Advisory Committee on Immunization is recommending the Pfizer-BioNTech vaccine for kids at this time. It says the risk of heart inflammation in Moderna's child sized dose is still unknown.



 Multiple Charges Laid after Thefts in Tillsonburg
Multiple Charges Laid after Thefts in Tillsonburg
 CASS Offering Unique Program to Students
CASS Offering Unique Program to Students
 PJHL Preview - Jan. 30th to Feb. 1st
PJHL Preview - Jan. 30th to Feb. 1st
 Woodstock Bowler Heading to Special Olympics
Woodstock Bowler Heading to Special Olympics
 Dog Bite Investigation in Tillsonburg
Dog Bite Investigation in Tillsonburg
 Interview with the Warden - January 29th, 2026
Interview with the Warden - January 29th, 2026
 Trevor Birtch Trial Daily Recap - Case 2
Trevor Birtch Trial Daily Recap - Case 2
 11 People Charged in Massive Drug Bust
11 People Charged in Massive Drug Bust
 Another Cold Warning for Oxford County
Another Cold Warning for Oxford County
 Chilly Charlie Returns on Monday
Chilly Charlie Returns on Monday
 Khanna Shines a Light on Oxford County Superstars
Khanna Shines a Light on Oxford County Superstars
 WFD Responds to Structure Fire
WFD Responds to Structure Fire
 WPS Appoints New Deputy Chief
WPS Appoints New Deputy Chief
 TMMC Woodstock Begins Production of Sixth Gen RAV4
TMMC Woodstock Begins Production of Sixth Gen RAV4
 Oxford OPP Briefs - January 28th, 2026
Oxford OPP Briefs - January 28th, 2026
 More Weather Alerts for Oxford County
More Weather Alerts for Oxford County
 Thrive Oxford Honoured at ROMA
Thrive Oxford Honoured at ROMA
 Tip Tuesday with the Oxford OPP - January 2026
Tip Tuesday with the Oxford OPP - January 2026
 Members Needed for Tillsonburg Housing Advisory Committee
Members Needed for Tillsonburg Housing Advisory Committee
 UPDATE: Ottawa Approves Plan to Move Marineland's Whales and Dolphins
UPDATE: Ottawa Approves Plan to Move Marineland's Whales and Dolphins



Comments
Add a comment